Monatomic Anion Names. The monatomic anions are named by adding -ide to the root of the name of the nonmetal that forms the anion. For example, N 3-is the nitride ion. The names of the anions are below. Hydride ion, H-nitride ion, N 3-phosphide ion, P 3-oxide ion, O 2-sulfide ion, S 2-selenide ion, Se 2- fluoride ion, F-chloride ion, Cl. .For atoms with LESS than 4valence electrons, they’re going to lose/give upelectrons to form positive cations.For atoms with MORE than 4valence electrons, they’re going to gain/stealelectrons to form negative anions.For atoms with 4 valence electrons, it can go either way.For atoms with 8 valence electrons, there is no change. Binary ionic compounds are made of cations (positively charged) and anions (negatively charged). Cations are formed by metals. Anions are formed by nonmetals. Some cations always have the same charge (fixed charge), and some have variable charges. It reflects the difference between the positively charged ions (called cations) and the negatively charged ions (called anions). An abnormal anion gap is non-specific—it does not diagnose a specific disease or illness—but it can suggest certain kinds of metabolic or respiratory disorders or the presence of toxic substances. Anions 1-acetate C 2 H 3 O 2-cyanide CN-amide NH 2-cyanate OCN-hydrogen carbonate fluoride F-(bicarbonate) HCO 3-hydride H-hydrogen sulfate hydroxide OH-(bisulfate) HSO 4-hypochlorite ClO-bisulfide HS-iodate IO 3-bisulfite HSO 3-iodide I.
Cation Definition
A cation is an atom or a group of atoms bearing one or more positive electric charges.
- Cations are formed in various ways, some of which are:
- When electrons are removed from neutral atoms or ions or other molecules.
- By the combination of positive ions with other molecules.
Se Cation Or Anion Cation
- By rupture of covalent when causes the shared paired of electrons is associated with one atom, and the other one becomes deficit.
- Cations are usually formed from metal atoms; however, positive radical ion might also have multiple atoms like in ammonium ion (NH4+).
- Cations are positively charged because they have more protons than electrons. Thus, cations are electron deficit.
- The size of cations is measured by measuring their ionic radius and cations, in general, have a smaller radius as they usually have one orbit less than their corresponding parent atoms.
- Hydrogen is the smallest cation that has no electron and thus is much smaller than its parent atom.
- In crystalline solids, the anions occupy most of the space in the lattice and cations are thus present between those spaces.
- Cations in the gaseous state are highly reactive and will react with anions to form neutral molecules. However, cations might be present in both liquid or solid-state.
- In the liquid state, cation interacts with the solvent to form solvated ions which are much stable.
- Because these are charged particles, their movements can be deflected by magnetic fields.
- Under an electric field, cations move towards the negative terminal (anode) to form neutral atoms. Most metals are purified by this process where they are platted onto the anode plate in an electric field.
- Cations are given different names to indicate the number of charges carried by these particles. Dications are cations with two positive charges, and trications are cations with three positive charges.
- Similarly, positively charged ions formed from organic molecules are termed carbocations.
Anion Definition
An anion is an atom or a group of atoms bearing one or more negative electric charges.
- Anions are formed in various ways, some of which are:
- When electrons are added to neutral atoms or ions or other molecules.
- By the combination of negative ions with other molecules.
- By rupture of covalent when causes the shared paired of electrons to be associated with one atom resulting in a negative charge.
- Anions are usually formed from non-metals; however, negative radical ions might also have multiple atoms like in sulfate ion (SO4—).
- Anions are negatively charged because they have more electrons than neutrons. Thus, anions are electron-rich.
- The size of ions is measured by measuring their ionic radius and anions, in general, have a larger radius as they usually have more electrons that repel each other creating a larger size than their corresponding parent atoms.
- Anions occupy most of the space in the crystal of solids as they have a larger size.
- Anions in the gaseous state are highly reactive and will react with cations to form neutral molecules. However, anions might be present in both liquid or solid-state.
- In the liquid state, anion interacts with the solvent to form solvated ions which are much stable.
- Under an electric field, anions move towards the positive terminal (cathode) to form neutral atoms.
- Most non-metallic gases are obtained by this process where they are collected from the positive terminal (cathode) of an electric field.
- Anions are given different names to indicate the number of charges carried by these particles. Dianions are anions with two negative charges, and trianions are anions with three negative charges.
- Similarly, negative ions formed from organic molecules are termed carbanions.
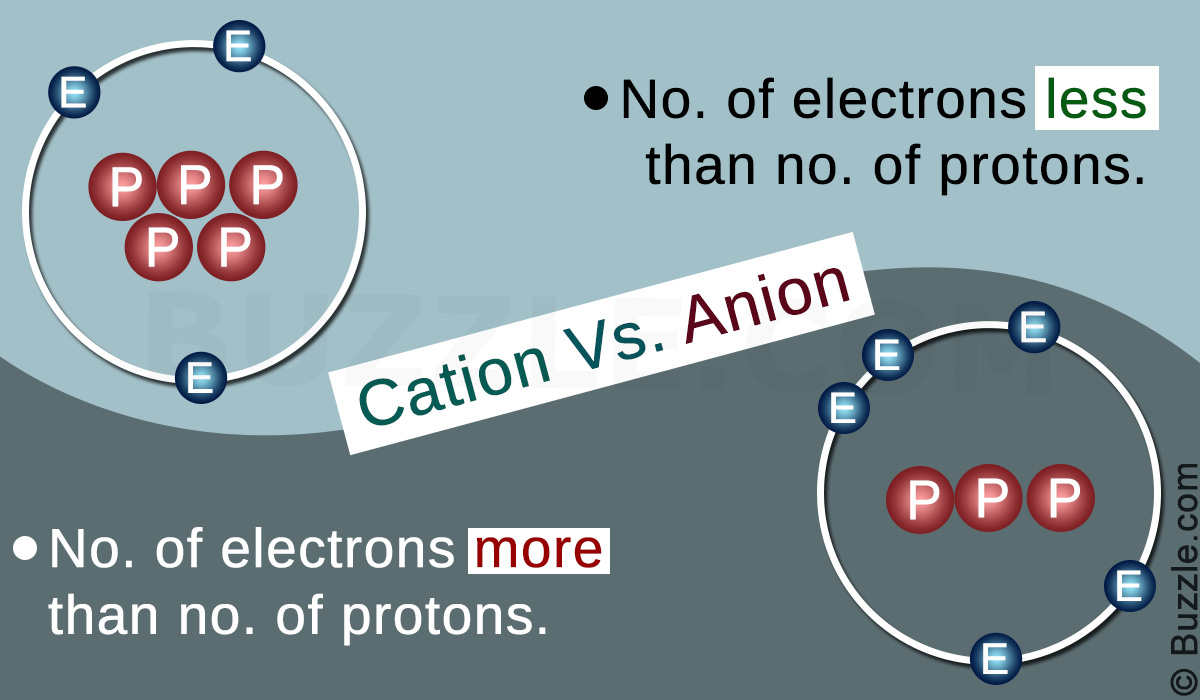
Key Differences (Cation vs Anion)
Basis for Comparison | Cation | Anion |
| Definition | A cation is an atom or a group of atoms bearing one or more positive electric charges. | An anion is an atom or a group of atoms bearing one or more negative electric charges. |
| Electric charge | Cations carry one or more positive charges. | Anions carry one or more negative charges. |
| Atoms | Cations are formed from metal atoms. | Anions are formed from non-metal atoms. |
| Electric field | Cations are attracted towards the negative terminal (anode) of an electric field. | Anions are attracted towards the positive terminal (cathode) of an electric field. |
| Reactions | Cations react with anions to form neutral molecules. | Anions react with cations to form neutral molecules. |
| Electrons | Cations have more protons than electrons. | Anions have more electrons than protons. |
| Size | Cations are smaller in diameter than anions. | Anions are larger in size than cations. |
| Organic ions | Organic cations are termed carbocations. | Organic anions are termed carbanions. |
| Crystal lattice | Cations occupy space between two anions (interstitial space) in the crystal lattice. | Anions occupy most of the space in the crystal lattice. |
| Examples | Some examples of cations are Na+, K+, NH4+, Ca2+, and Al3+. | Some examples of anions are SO4—, Cl–, F–, PO4—and I–. |
Example of Cation
Sodium-ion
- Sodium atom is a monoatomic monocation that is formed from the ionization of sodium atom.
- Sodium is a metal that during the breaking of bonds, gives the shared pair of electrons to the anion, thus carrying a positive charge.
- The molecular formula of sodium ion is Na+ with an ionic radius of 0.102 nm.
- Sodium ions are necessary for several physiological activities in the body like regulation of body fluids like blood, the transmission of nerve impulses, heart activity, and other metabolic functions.
- Sodium is important in other animals, as it is maintained at a high concentration in their blood and other extracellular fluids, but the ion is not necessary for plants.
- Humans require less than 500 mg per day of sodium in their diet.
- However, in people with salt-sensitive blood pressure, extra intake of sodium may cause a negative effect on health.
Example of Anion
Chloride ion
- Chloride ion is a diatomic monoanionic that is formed from the ionization of the chlorine atom.
- Chlorine is a non-metal that during the rupturing of bonds, takes the shared pair of electrons, resulting in a negative charge.
- The molecular formula of the chlorine ion is Cl– with an ionic radius of 0.181 nm.
- Chloride ion is an essential electrolyte present in almost all body fluids. It is responsible for transmitting nerve impulses, maintaining acid/base balance, and regulating fluid in and out of cells.
- The kidneys carefully control the number of chloride ions in the blood.
- Chloride-transporting proteins (CLC) are a particular type of protein that plays fundamental roles in many tissues, in the cell membrane as well as in intracellular membranes.
- CLC proteins form a gene family that comprises nine members in mammals, at least four of which are involved in human genetic diseases.
References and Sources
- National Center for Biotechnology Information. PubChem Database. Sodium-ion, CID=923, https://pubchem.ncbi.nlm.nih.gov/compound/Sodium-ion (accessed on July 13, 2020)
- National Center for Biotechnology Information. PubChem Database. Chloride ion, CID=312, https://pubchem.ncbi.nlm.nih.gov/compound/Chloride-ion (accessed on July 13, 2020)
- 3% – https://www.ncbi.nlm.nih.gov/pubmed/17729441
- 1% – https://www.worldatlas.com/articles/what-is-the-difference-between-a-cation-and-an-anion.html
- 1% – https://www.emedicalprep.com/study-material/chemistry/solid-state/interstitial-sites-close-packing/
- 1% – https://www.britannica.com/science/ion-physics
- 1% – https://www.bbc.co.uk/bitesize/guides/ztc6w6f/revision/1
- 1% – https://pubchem.ncbi.nlm.nih.gov/compound/Sodium-ion
- 1% – https://en.wikipedia.org/wiki/Anion
- 1% – https://answersdrive.com/what-does-chloride-do-for-water-4388216
- <1% – https://www.thoughtco.com/definition-of-anion-and-examples-604344
- <1% – https://www.enotes.com/homework-help/why-electrons-flow-from-cathode-anode-during-flow-686303
- <1% – https://sciencestruck.com/difference-between-cation-anion
- <1% – https://en.wikipedia.org/wiki/Cations
- <1% – https://en.wikipedia.org/wiki/Cationic
- <1% – https://en.wikipedia.org/wiki/Amidogen
- <1% – http://www.chemistry.wustl.edu/~edudev/LabTutorials/CourseTutorials/LabTutorials/Dialysis/Kidneys.html
Using ion exchange resins for industrial water purification and separation can be complex, especially for those unfamiliar with what ion exchange resins are and how they work. If you are looking for a general explanation of “what the differences are between cation and anion exchange resins,” the two most-used resins in ion exchange technology, this article simplifies the similarities and differences and outlines some fundamental information you should know when seeking to understand these ion exchange basics.
How cation and anion exchange resins are similar
Cation and anion exchange resins are both small, porous, plastic beads (approximately .5 mm diameter, which varies) that are fixed with a specific charge. This “fixed” charge cannot be removed and is part of the resin’s crosslinked makeup or structure. Each resin bead must also contain a neutralizing counterion that is able to move in and out of the bead, which is replaced with an ion of similar charge during the process of ion exchange (when an aqueous solution is passed through the beads and the ion exchange occurs, removing the undesirable contaminant).
How cation and anion exchange resins are different
The main difference between cation and anion resins is that one is positively charged (cation) and the other is negatively charged (anion). This makes them useful in removing different types of contaminants (which will also vary depending on their size and chemical composition). Cation and anion resin beads can be used together (mixed bed configuration) or in separate vessels (twin bed configuration), depending on the needs of the facility and if total removal of positively and negatively charged ions are required.
Although anion and cation exchange resins are the main two categories of resins used in ion exchange, there are four main types for standard water treatment that include:
- Strong base anion
- Weak base anion
- Strong acid cation
- Weak acid cation
Below is a general overview of what each of these types of resins are:
Strong base anion resins
Strong base anion (SBA) exchange resins are typically used for demineralization, dealkalization, and desilication, as well as removal of total organic carbon (TOC) or other organics depending on the type of resin. They are available in multiple varieties, each of which offer a unique set of benefits and constraints, but in general, SBA resins are strong enough to remove both strong and weak acids (including carbonic and silicic acid).
Weak base anion resins
Weak base anion (WBA) exchange resins are often paired with SBA units for demineralization applications as they only remove anions associated with stronger acids (like chloride and sulfate) and will not remove weak acids (like carbon dioxide and silica). This can be beneficial for facilities that wish to remove the stronger acids while leaving the weaker behind, but commonly, WBA and SBA are often used jointly to complete a more thorough demineralization process.
Strong acid cation resins
Strong acid cation (SAC) exchange resins are among the most widely used resins, especially for softening applications, as they are effective at complete removal of hardness ions such as magnesium (Mg+) or calcium (Ca2+). Certain varieties of SAC resins have also been developed for applications demanding removal of barium and radium from drinking water or other streams. SAC resins can be damaged by oxidants and fouled by iron or manganese, so care must be taken to avoid exposure of the resin to these materials.
Weak acid cation resins
Weak acid cation (WAC) exchange resins remove cations associated with alkalinity (temporary hardness) and are used for demineralization and dealkalization applications. Additionally, WAC resins tend to have relatively high oxidation resistance and mechanical durability, making them a good choice for streams containing oxidants such as hydrogen peroxide and chlorine.
How SAMCO can help
Cation And Anion Chart
SAMCO has over 40 years’ experience custom-designing and manufacturing ion exchange systems and providing ion exchange resins for a range of industries and solutions, so please feel free to reach out to us with your questions. Some of our most innovative solutions come in the form of the various resin technologies we offer. Our resins cab be extremely effective in the removal of hardness, alkalinity, chloride, mercury, and organics, to name a few.
We are also the Northeast licensed distributor of AMBERPACK™ and UPCORE™ technologies by The Dow Chemical Company (formerly Rohm and Haas). These are two of the most advanced ion exchange systems available today.
List Of Cations And Anions
For more information or to get in touch, contact us here to set up a consultation with an engineer or request a quote. We can walk you through the steps for developing the proper solution and realistic cost for your ion exchange treatment system and resin needs.
Cation Anion Calculator
To learn more about SAMCO’s innovative technologies and services, visit our innovations page here.
Cl Cation Or Anion
If you want to learn more about ion exchange resins, these other articles might be of interest to you: